Chronic Rhinosinusitis (CRS) is a long-term sinus disease affecting 1 in 10 adults in the UK. Symptoms of CRS include a blocked and runny nose, loss of smell, facial pain, tiredness and worsening of breathing problems, such as asthma. Studies have shown that sinus disease can have a greater impact on quality of life than heart disease and back pain.
by the time I went to see the consultant I was armed and ready to do battle. I hadn’t got this far to be told ‘my sense of smell wasn’t that important’ or ‘at least I can breathe’ or ‘that research is unfounded’ or ‘I’ve never heard of that problem’ – basically all the things I’d heard for 25+ years.
The type of treatments given by GPs and Ear, Nose and Throat specialists in the NHS varies greatly. This is because there is currently little evidence to show the comparative effectiveness of the treatments used to manage CRS and a limited understanding of which type of patients benefit most from each of the treatments available.
The MACRO Trial, which is part of a large programme of work funded by the government, aims to answer these questions with a view to establishing guidelines that will allow for a greater consistency and effectiveness in the treatment of adult CRS patients within the NHS.
Below MACRO's Chief Investigator, Professor Claire Hopkins, explains the limitations in current practice for treating CRS, how the MACRO trial aims to address these limitations and what participation in the MACRO trial will entail.
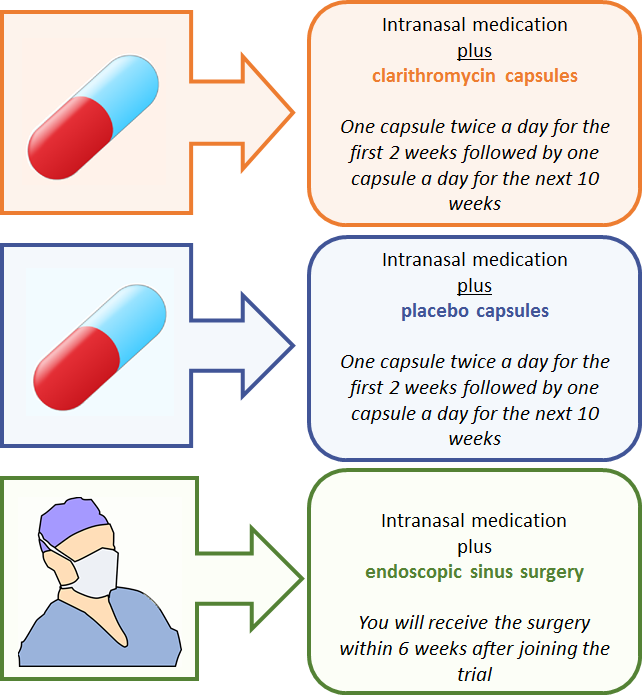
The MACRO trial will investigate three different treatment options for patients with CRS.
All participants who join the MACRO trial will be asked to use standard care (intranasal medications), which are considered the current best practice for management of CRS. Intranasal medication includes nasal steroid sprays/drops and saline rinses.
In addition to intranasal medication, each participant will be randomly allocated to receive one of three treatment options: clarithromycin capsules, placebo capsules or endoscopic sinus surgery. Each participant will have an equal chance of getting one of these three treatment options.
Please click here if you'd like to download a copy of the MACRO Trial Patient Information sheet, which will provide you with lots of information about the trial.
If you are interested in joining the trial, you can find more information on the on the Interested in Participating in the MACRO Trial page.
If you are already a participant in the MACRO Trial, further information and news about MACRO can be found on the Already a MACRO Participant page.

